Sampling Protocols:
Materials, Site Selection & Best Practices
What equipment do I need?
Data Collection Equipment
To collect data, you will need a Smartphone or Tablet[1] and the ArcGIS Survey123 app by esri. Other optional tools include: iNaturalist, Seek by iNaturalist, or PictureThis for plant identification.
[1] Apple devices tend to work more reliably with the apps.
Sample Collection Equipment
All gear required for sampling can be sent to you in sample collection kits. Please reach out to: ckamoroff@stillwatersci.com for more information and coordination.
For a comprehensive list of equipment for each sampling type, please see the respective sampling protocol.

How do I collect the data?
Install the app and download the survey for offline use BEFORE going into the field!
Download the Sampling Survey here: https://arcg.is/5naKi0 or use this QR code.
Open the Survey123 app (preferred) OR continue using the web on your smartphone or tablet (ensure that location data and access to photos are enabled).
Open the “Soil Sampling Survey” and click to Collect.
Date and Time:
Date and time will auto-populate the ‘+’ icon to the current date and time. If entering information from previously collected data, click the ‘x’ icon and enter the correct date and time of sample collection.Site ID:
If sampling site has a site ID, enter it (optional).Observers:
Include first and last names of all observers. List primary observer first.Phone Number:
Include primary observer’s email and/or phone. This will only be used to contact the primary observer if there are questions regarding sampling collection or data entry.Plot coordinates of the site from approximately the central point of your sampling area using the Survey123 form. Ecoregion should automatically populate. If not using a smartphone, the smartphone’s GPS is not working, or you are entering information that was previously collected, check “No” for “GPS working?” and then manually enter the Latitude and Longitude of the central point. If manually entering location information, please include accuracy, coordinate system, or other pertinent information in the “Location notes” field.
Using the Survey123 app, take photos of all cardinal directions from the central point of the site.
Include pertinent notes such as deviations from the protocol, dominant plant species, habitat type, site slope (e.g. “flat” or “steeply sloping to the north”), amount of bare ground versus plant cover, unique features of the site, etc.
Check the box for each sample type collected during the visit (i.e., litter, soil eDNA, bulk soil, or insect sample).
Before scanning each barcode, make sure all other information has been entered, otherwise site data will not be associated with that sample ID when uploaded. Scan each barcode associated with each sample. There should be separate barcodes for each type of sample collected (e.g., bulk soil will have one sample and barcode while the insect sample will have a separate barcode)
Note whether the barcode function is working (Yes or No). If the barcode function is not working or if you do not have a barcode, please enter the barcode ID or sample ID in by hand. If accessing the survey form via the web and not the Survey123 app., the barcode function may not work.
Be sure to review the sample and barcode label data for accuracy, making sure the sample type/sample ID summary at the bottom of the form correctly describes the samples you collected. Once the data has been reviewed, select “yes” under “Field data QAQC’d” and click the check mark.
If you are connected to WIFI or have service, click “Send now.”
If you are not connected to WIFI and do not have service, click “Save to Outbox” and send once WIFI or service connections can be made.
Where do I sample?
Collect samples in natural areas that are not impacted by anthropogenic disturbances.
If possible, collect leaf litter and soil samples away from roads, trails, etc. To prevent the spread of pathogens DO NOT collect leaf litter or soil in agriculture/ agricultural fields. It is fine to collect insect samples along roads or trails and in agricultural fields. For agriculture focused project, please contact ckamoroff@stillwatersci.com.
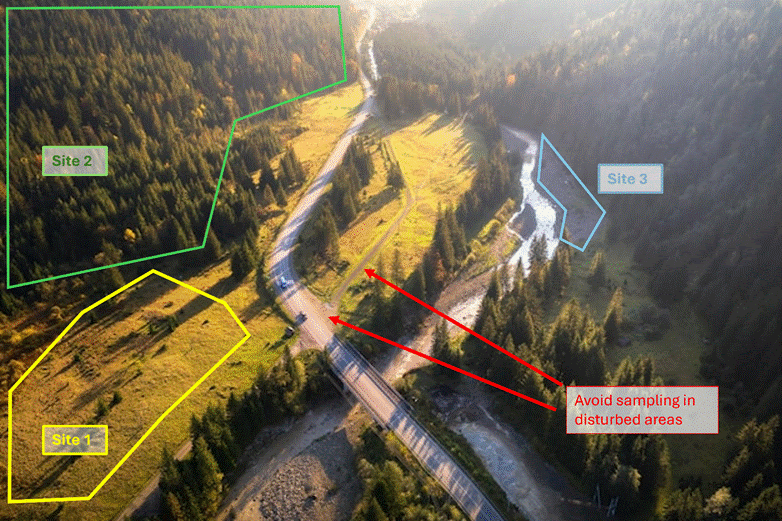
Collect samples within a sampling site. A sampling site is an area of similar habitat with uniform vegetation types and where environmental conditions are consistent throughout (e.g., a meadow or forested area). One sample site should be smaller than 0.25-mile (0.4 km). See Figure 2-1 for example sample sites. Collect from areas across the site that reflect the site’s biodiversity (e.g., sample under a variety of plant types or soil types present at the site).
Figure 2-1. Example of Sample Sites.
What permits do I need?
If you are collecting samples on private property, you need to obtain permission from the landowner prior to sampling. Please be sure to document landowner permissions.
The ATBI team has assembled collection permits and access permissions for other landowner groups. Use the CalATBI Logistics Map to assess a location where you would like to collect data and view available permits.
If you are collecting non-listed terrestrial insects and need a California Department of Fish and Wildlife Scientific Collecting Permit, please contact ckamoroff@stillwatersci.com.
Partners collecting insects under the ATBI California Department of Fish and Wildlife Scientific Collecting Permit are expected to understand and comply with its terms.
What do I do with the samples after collection?
While in the field, it is best to store samples in coolers with ice packs (do not use regular ice as it could melt and contaminate samples). Sealed, frozen bottles of water work well as field ice packs. It is recommended that you place samples or ice packs inside a large leak-proof bag to prevent any contact with meltwater. Samples can also be stored in a fridge. Do not freeze samples.
If you do not have access to coolers, ice packs or fridge, store samples in an insulated bag (e.g., wrap samples in a sleeping bag or sweater and store in a backpack) and keep the bag out of the sun.
To maximize the amount of biodiversity extracted from each sample, ship or drop off soil and leaf litter samples as quickly as possible, ideally within 24-48 hours after collection. The shortest time between collecting the samples and sample processing maximizes the number of species that can be identified in each sample. If leaf litter or soil samples are held for long periods of time, for example 1 to 2 weeks, they can still be processed. Do not throw away older samples.
Insect and soil eDNA samples can be stored in a fridge for 2 to 4 weeks. Ship or drop off insect and eDNA samples at your earliest convenience.
Shipping labels and shipping instructions will be sent with sampling kits. If additional support is needed, please coordinate with ckamoroff@stillwatersci.com.
How do I prevent the spread of pathogens?
To minimize the spread of insects, disease, and noxious weeds, it is important to clean all equipment that comes in contact with soil between sampling sites. Before leaving the site and/or before sampling a new site, remove dirt, mud, and vegetation from boots, spade, and any other gear used during sampling. Please also remove any seeds or plant matter from your sweep net. When possible, bleach tools with bleach wipes or 10% bleach solution in-between sites.
To reduce the likelihood of disease transmission in bumble bees, if you accidentally capture a bumble bee, decontaminate collection equipment. For nets or other mesh sampling materials soak in a 10% bleach solution for 20 minutes and rinse with water or launder with bleach. Spray other non-mesh equipment with diluted bleach. To enhance effectiveness of sanitation treatments, ensure bleach is new and has not been degraded by sunlight.
Thank you for doing your part in limiting the spread of invasive species and diseases.